Week 9. Plant Nutrient and Nutrient Cycle
Introduction
Plants require only light, water, and about 20 elements to support all their biochemical needs. These 20 elements are called essential nutrients. For an element to be regarded as essential, three criteria are required:
- a plant cannot complete its life cycle without the element
- no other element can perform the function of the element
- the element is directly involved in plant nutrition
Macronutrients and Micronutrients
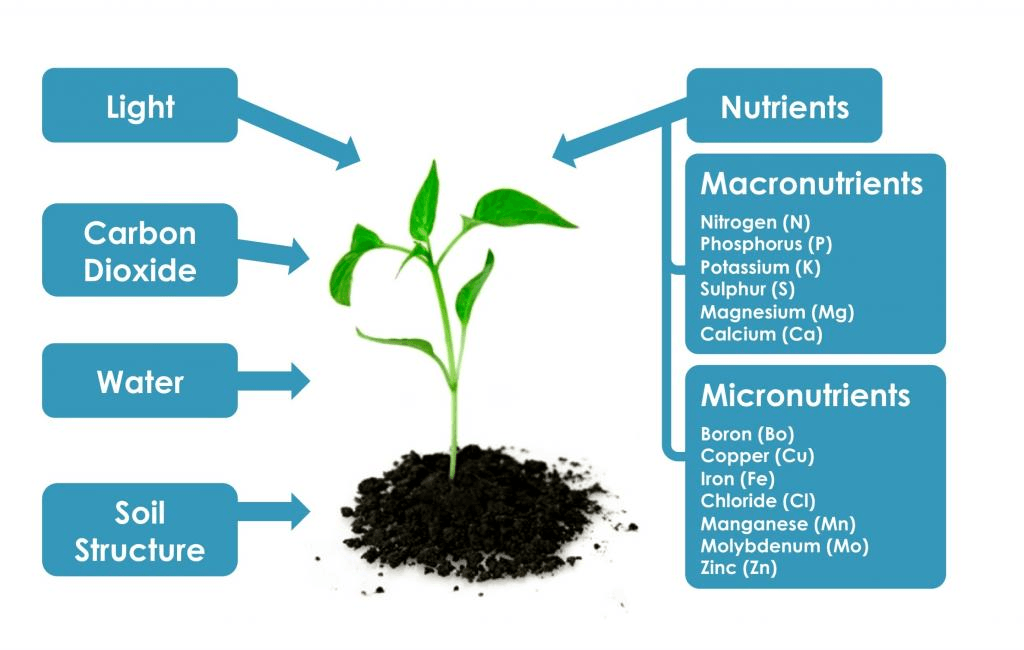
The essential elements can be divided into macronutrients and micronutrients . Nutrients that plants require in larger amounts are called macronutrients.
About half of the essential elements are considered macronutrients: carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur.
Sources, Functions and Deficiency Symptoms of Essential Elements in Plants
The seven macro nutrients in plants are: (1) Carbon, Hydrogen and Oxygen (2) Nitrogen (3) Phosphorus (4) Potassium (5) Calcium (6) Magnesium and (7) Sulphur.
Carbon, Hydrogen and Oxygen (C, H and O):
These are the non-mineral essential elements commonly enter a plant body as CO2, H2O. These elements are the building blocks of macromolecules and constitute over 90% of the total dry matter of the plant. Hence these are commonly known as framework elements.
Nitrogen (N):
Source:
Plants absorb nitrogen from the soil in three forms: nitrate (NO3- ), Nitrite (NO2-) or Ammonium (NH4+).
Regions of requirement:
Nitrogen is required in all plant parts particularly in meristematic tissues.
Functions:
(i) It is the major constituent of proteins, purines, pyrimidines, vitamins, cholorophyll and hormones,
(ii) It is also present as a component of coenzymes like FAD, NAD, NADP etc.
(iii) Older leaves when turn yellow, their nitrogen passes to younger parts in the form of amines and amides.

Fig – Nitrogen Deficient leaves
Deficiency Symptoms:
(i) Stunted growth due to reduced cell division and dormant lateral buds.
(ii) Chlorosis (yellowing of leaves),
(iii) Suppressed or late flowering,
(iv) Increase in starch content but decrease in protein content,
(v) Wrinkling of cereal grains,
(vi) Purple colouration appears in shoot axis.
Phosphorus (P):
Source:
Soil contains phosphorus in organic and inorganic forms. Plant absorbs only inorganic forms: monovalent phosphate anions (H2PO4) and divalent phosphate anions (H2PO4)2-. The organic forms will be available only after transformation into inorganic forms.
Regions of requirements:
Used mostly in younger tissues.
Functions:
It is a constituent of phospholipids (membrane lipids), nucleic acids, nucleotides, coenzymes, ATP, metabolic intermediates, sugar phosphates in photosynthesis etc.

Fig – Phosphorus deficient leaves
Deficiency Symptoms:
(i) Purple or red pigmentation on leaves
(ii) Premature fall of leaves and floral buds
(iii) Delay in seed germination
(iv) Older leaves affected first and become dark brown,
(v) Stunted and slender stem in young plants,
(vi) Accumulation of carbohydrates in Glycine max (Soybean),
(vii) Vascular tissues reduce in tomato plants.
Potassium (K):
Source:
It is absorbed as potassium ion (K+).
Regions of requirements:
Required in meristematic tissues, leaves and root tips. It accumulates in older leaves.
Functions:
(i) Essential for stomatal opening, translocation of sugar, protein synthesis, activation of enzymes and maintenance of cell turgor.
(ii) Determine anion-cation balance in cell.

Fig – Potassium deficient leaves
Deficiency Symptoms:
(i) Mottled or marginal chlorosis followed by necrosis of leaf tips, margins and between veins
(ii) Loss of apical dominance, leads to rosette or bushy habit
(iii) Loss of cambial activity
(iv) Disintegration of plastids
(v) Rate of respiration increases
(vi) Dieback of shoots i.e. progressive death from shoot tip towards base
(vii) Increased tendency to lodging (bent to the ground) in corn.
Calcium (Ca):
Source:
Plant absorbs calcium from the soil in the form of Ca2+ ions. Deficient in sandy soils.
Regions of requirements:
Meristematic and differentiated tissues; accumulated in older leaves.
Functions:
(i) Used in synthesis of calcium pactate in middle lamella of cell wall
(ii) Involved in normal functioning of cell membrane
(iii) Used in formation of mitotic spindle
(iv) Serve as a second messenger in action of phytohormones.
(v) Activates certain enzymes like ATPase, kinases and succinate dehydrogenase.

Fig – Calcium deficient leaves
Deficiency Symptoms:
(i) Stunted growth
(ii) Chlorosis, downward hooking and deformation in young leaves,
(iii) Necrosis of young meristematic regions such as root tips or young leaves.
Magnesium (Mg):
Source:
Like calcium, magnesium is also available in the soil in form of exchangeable cation. It is absorbed as divalent Mg2+.
Regions of requirements:
It is required in leaves. It is withdrawn from ageing leaves and exported to developing seeds.
Functions:
(i) Important constituent of chlorophyll
(ii) Maintains ribosome structure and chromatin fibre
(iii) Activates enzymes in respiration, photosynthesis and synthesis of DNA and RNA.

Deficiency Symptoms:
(i) Chlorosis between the leaf veins
(ii) Necrotic or purple spots on older leaves.
(iii) Premature leaf abscission
(iv) Extensive development of chlorenchyma and scanty pith formation.
Sulphur (S):
Source:
Plants obtain sulphur from soil as divalent sulphate anions (SO42-). Atmospheric SO2 and SO3 are also absorbed directly.
Regions of requirements:
In plants sulphur is required in stem, root tips and young leaves.
Functions:
(i) It is a constituent of amino acids like cysteine, cystine and methionine,
(ii) It is also the main constituents of Coenzyme A, Vitamins (thiamine and biotin), ferridoxin etc.
(iii) It is essential for stabilizing the structure of protein by formation of disulfide bond (S-S) between two cysteine residues to form a cystine,
(iv) Characteristic pungent odour of mustard, onion, garlic etc. is due to presence of sulphur containing volatile oils.
Deficiency Symptoms:
The sulphur deficiency symptoms are similar to those of nitrogen deficiency because sulphur and nitrogen are constituents of proteins.

Sulphur deficiency causes:
(i) Chlorosis of younger leaves
(ii) Stunted growth
(iii) Accumulation of anthocyanin
(iv) Terminal bud growth in inhibited
(v) Lateral buds develop prematurely.
Iron (Fe)

Function:-Iron is an essential element required for the synthesis of chlorophyll. It is involved in the activation of many enzymes used in photosynthesis and respiration.
Iron is relatively immobile and is generally in short supply in alkaline soils.
Symptoms of Deficiency:-Young leaves develop chlorosis in the interveinal areas which may develop into white leaves with necrotic spots. Stunted growth.
Manganese (Mn)

Function:-Manganese is an essential nutrient for the growth of both plants and animals. In plants it enhances root growth, disease resistance and the development of fruit. It is required for the synthesis of chlorophyll and assimilation of nitrate. It is involved in the activation of many enzymes involved in photosynthesis and respiration.
Symptoms of Deficiency:-Symptoms vary with species; in cereals – grey – white spots, flecks and stripes may appear in the interveinal areas. In legumes, interveinal chlorosis of young and middle aged leaves and tissue may rapidly become necrotic. Seed disorders e.g. “split seed” or “marsh spot” may develop.
Boron (B)

Function:-
Boron is an essential element in plant nutrition. It is essential for root tip, pollen tube and shoot growth and the synthesis of DNA and RNA.
Symptoms of Deficiency:-
Leaf blades may be distorted and stems may become brittle and crack e.g. “stem crack” in celery. Shorter intermodal length, retarded growth or necrosis of the terminal buds and youngest leaves. Reduction or failure to seed and fruit. Malformation of fruit.
Zinc (Zn)

Function:-
Zinc is an essential nutrient required for the functioning of a large number of enzymes involved in the growth and reproduction of both plants and animals. It is required for the synthesis and functioning of chlorophyll, is involved in the plant hormone system and as a catalyst for the plant growth regulator, auxin.
Symptoms of Deficiency:-
In plants, shortened internodes with excessive branching (resetting) of small, dark green deformed leaves. In cereals and grasses – chlorotic bands (yellow, red) may appear either across or within the veins. Stunted growth and necrosis of older leaves.
Copper (Cu)

Function:-
Copper is an essential nutrient required for the functioning of a large number of enzymes involved in the growth and reproduction of both plants and animals. It is required for the synthesis and functioning of chlorophyll, is involved in the plant hormone system and acts as a catalyst for the plant growth regulator, auxin.
Symptoms of Deficiency:-
Young leaves become dark green, twisted and deformed. Necrotic spots may appear. In grains and grasses, seed production is reduced and seed heads may be white and empty.
Molybdenum (Mo)

Function:-Molybdenum is an essential element for both plants and animals. In plants, Molybdenum is required for protein synthesis.It enhances both photosynthesis in plants and nitrogen fixation in legumes.
Symptoms of Deficiency:-In plants, reduced and irregular leaf blade formation, interveinal mottling and chlorosis around the edges of older leaves. Necrotic spots at leaf tips and edges, smaller root nodules coloured white or green (not pink),growth inhibition in legumes.
Cobalt (Co)
Function:-Cobalt is a key constituent of Vitamin B12 and Propionate (themajor source of energy in ruminants). Cobalt enhances the nitrogen fixing ability of legumes and improves the efficiency of ruminal digestion.

Symptoms of Deficiency:-
Small root nodules on legume species.
Uniformly pale green – yellow leaves, most severe on old leaves. Some crops may develop red leaves, stems or petioles. Stunted growth – tops may be less leafy. Grain or seed production may be retarded.
Symptoms of Deficiency:-White muscle disease, ill-thrift, stiff lamb disease, infertility and embryonic mortality.
Factors Affecting Availability of Soil Nutrients
The following are the factors that influence the availability of nutrients in the soil:
- Soil pH
- Concentration of other nutrients
- Leaching
- Burning
- Crop Removal
- Oxidation
- Erosion
Soil pH
This describes the degree of acidity or alkalinity of the soil or any other medium in which a plant is grown. The level of such acidity or alkalinity affects the availability of nutrients in both the soil and the plants in the following ways:
- At low pH (high acidity), several micronutrients such as iron, manganese and zinc are dissolved in high quantity. Such trace elements that are required in small quantities are released in excess, causing toxicity to plants.
- Low pH (increased acidity) also hinders the activities and sometimes kills beneficial soil organisms that help in organic matter decomposition and nitrogen fixation.
- High acidity also encourages the breakdown of clay minerals like calcium, iron and aluminium, causing them to leach away from the soil.
- Low pH also reduces the availability of some nutrient elements like nitrogen, phosphorus and sulphur. Phosphorus is more available at a pH of 6–7.
- At high pH, calcium and magnesium ions accumulate in the soil and this affects plant growth negatively.
Concentration of Other Nutrient Elements
Availability of different nutrients depends on the concentration of other elements in the soil. The nutrients therefore need to be balanced if anything good would be obtained from such soil. For example, excess amount of soluble iron and aluminium in acidic soils and calcium in alkaline soil reduces the availability of phosphorus. Thus, excess amount of some elements prevent proper absorption and utilization of other elements; for example, high concentration of nitrogen and phosphorus in soil results in non-availability of potassium. These conditions result in retarded growth, low yield and eventual death of the plant.
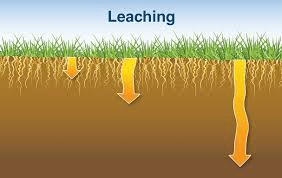
Leaching
This is the washing down of nutrients in soluble form from the plant root zone down the soil beyond the reach of plants’ roots. Nutrients such as calcium, magnesium and potassium are lost from the topsoil this way. Leaching causes the accumulation of aluminium and hydrogen ions which later becomes acidic and toxic to plants.
Burning
It destroys soil organic matter directly and exposes the soils to erosion and other factors of the environment that wash or sweep away soil organic matter. Organic matter contents are very rich in nitrogen, phosphorus and sulphur but these nutrients are lost in gaseous form during burning. Burning also destroys beneficial soil organisms that help in soil aeration and organic matter decomposition.

Crop Removal
Plants utilise the nutrients taken from the soil to build up their body tissue and to effect growth, development and production. At harvesting, the whole plant is removed away from the field and consumed; thus the nutrients used up by the plants are taken away and not returned to the soil. The rapid removal of nutrients this way by continuous cropping completely depletes the soil of such nutrients.
Oxidation
It involves change in valency. Some nutrients like manganese and iron exist in reduced or oxidised forms. The oxidized form of manganese and iron (Fe3+) is not soluble, thus reducing their availability to plants. Also some compounds such as that of ammonia are oxidised to gaseous ammonia. Nitrates are also reduced to molecular nitrogen or oxides of nitrogen by denitrifying bacteria. These products escape into the atmosphere and the soil is depleted of the nutrients.
Erosion
Rainfall causes water erosion by washing away the topsoil along with its nutrients. The top soil is also blown away by wind along with the nutrients contained in it.

Methods of Replenishing Lost Soil Nutrients
The following are common ways by which farmers replenish the soil or replace the nutrients that have been lost in the soil.
Crop Rotation
This is the system of farming in which the farmer grows different crops on the same piece of land year after year in a definite order so as to maintain the soil fertility. The rotation is planned in such a way that nutrients taken from soil during crop removal are restored.
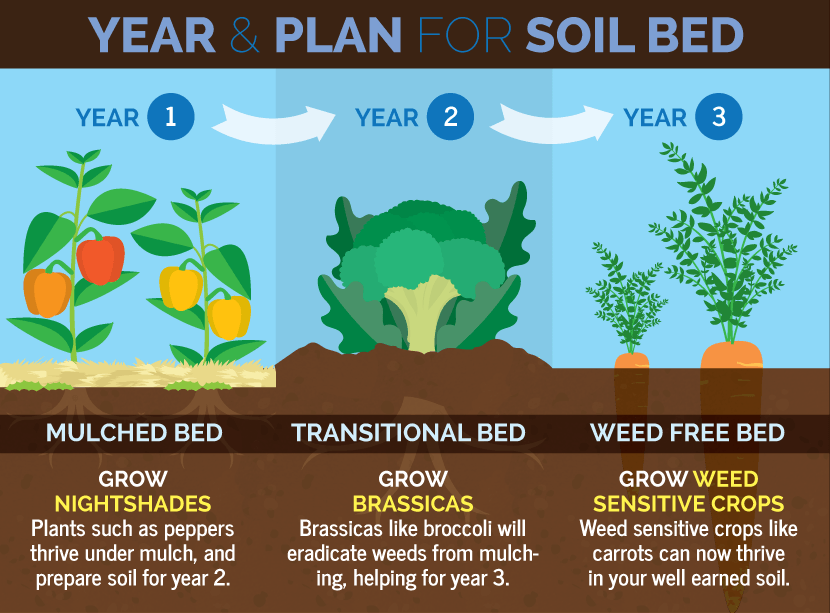
Fallowing
This is much like shifting cultivation. It is a method where the soil is left uncultivated for a relatively short period after cultivation for about 2 years before it is cultivated again. During the period, the soil is left to fallow. The vegetation that grows on it is later turned into the soil and ploughed over to supply nutrients. This is different from shifting cultivation because the period of fallow is shorter and the farmer may not relocate to another site.
Organic Manuring
Manure is any material that increases the fertility of the soil when added to it. Organic manure relates to the materials that are derived from either plant or animal origin or a combination of both which have decomposed and would readily release the nutrients they contain. There are three major types:
-
- Farm-yard manure
- Compost manure
- Green manure.
(a) Farm-yard manure This is a collection of animal wastes such as animal dung, faeces or poultry droppings, urine, beddings which have been allowed to undergo series of decomposition before being used as a fertilizer.
(b) Compost manure This is the type of manure that is prepared by deliberate action of human beings such that organic materials are packed together in a definite order or series and allowed to decompose progressively under careful supervision. Compost manure can be prepared in two ways.
- The pit method: This type is suitable for savanna areas where there is low rainfall.
- The heap method: This is suitable for the forest areas where rainfall is high.
(c) Green manure This is formed from leguminous cover crops and other fresh growing plants which have been ploughed into the soil while they are still green and fresh in the field. These plants are ploughed under when they are still young before flowering so that the rate of decomposition is fast. Examples of leguminous plants for green manures are cowpea, Centrosema, Calopogonium, Pueraria and grasses.
Environmental Cycles (Nitrogen Carbon Water And Phosphrus Cycle)
A natural process in which elements are continuously cycled in various forms between different compartments of the environment (e.g., air, water, soil, organisms).
Examples include the carbon, nitrogen and phosphorus cycles (nutrient cycles) and the water cycle.
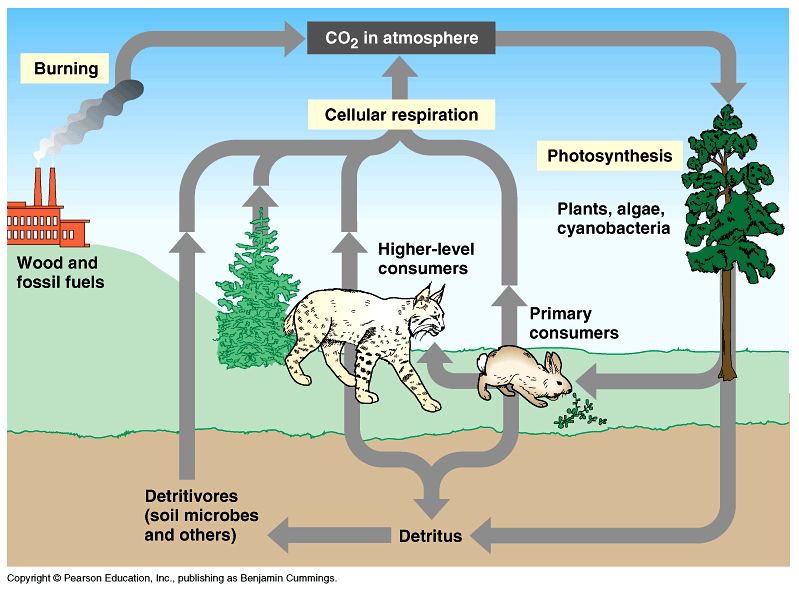
The carbon cycle includes the uptake of carbon dioxide by plants through, its ingestion by animals and its release to the atmosphere through respiration and decay of organic materials. Human activities like the burning of fossil fuels contribute to the release of carbon dioxide in the atmosphere.
The Carbon Cycle Starts with Carbon in the atmosphere, plants pull the Carbon dioxide out of the air through photosynthesis. Consumers eat plants, and digest parts of the carbon. through respiration, consumers release them back into the atmosphere. When the animal dies off, it eventually evolves into fossil fuels, which is made up largely of carbon. Humans use the fossil fuels and release the carbon into the air.
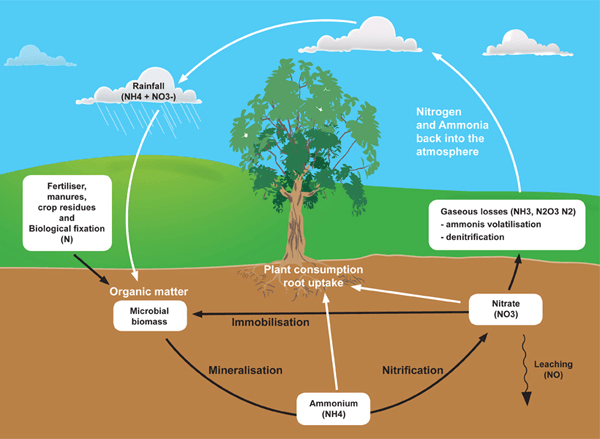
The nitrogen cycle involves the uptake of nitrogen form the atmosphere by a process called fixation which is carried out by microbes or industrial processes. Decomposition of biological waste by microbes can return nitrogen to the atmosphere. Nitrogen is mainly used by humans as a fertilizer in farmlands, but its excessive usage can lead to serious problems (such as eutrophication).
The Nitrogen Cycle Starts with Nitrogen in the atmosphere. The nitrogen gets into the ground through nitrogen fixation (Lightning & Bacteria. Consumers consume plants that absorb the nitrogen from the soil, and when the animal dies and decays, the nitrogen is released from its body and is absorbed back in the soil. We use the nitrogen in fossil fuels to make fertilizers. When it rains, the Nitrogen is washed away (runoff) into surface water, or be absorbed into the ground (Ground Water). The runoff of the nitrogen into the surface water is that it destroys all the oxygen build up in the water, called Eutrophication.

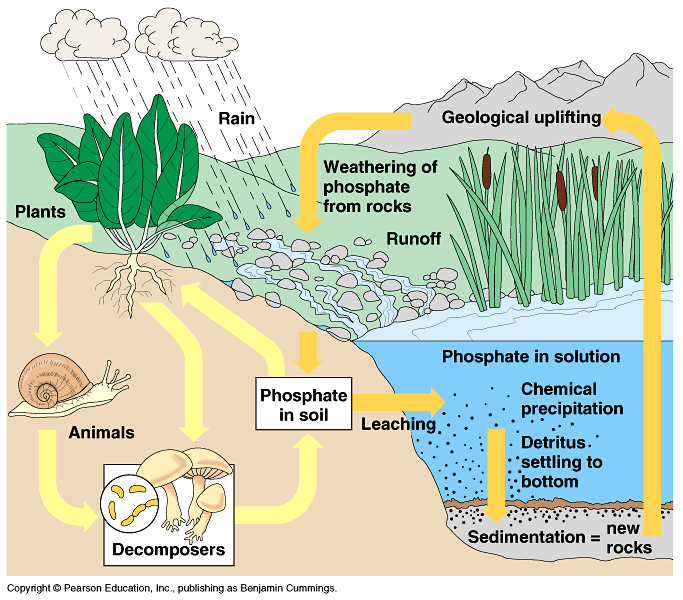
The phosphorus cycle involves the uptake of phosphorus by organisms. Phosphorus in the environment is mainly found in rocks, and natural weathering processes can make it available to biological systems. After decomposition of biological waste, it can accumulate in large amounts in soils and sediments. Phosphorus is used by humans as a fertilizer in farmlands and in detergents. Overuse of phosphorus can lead to eutrophication.
The Phosphorus Cycle starts with the weathering of rocks. This releases phosphorus into the ecosystem. The phosphorus is absorbed by plants, which are consumed by animals. When the plants or animals die, they release the phosphorus back into the soil through decomposition. Through mining, we use the phosphorus and put it into fertilizers, which is once again consumed by the ground, and released into surface water. Which causes Eutrophication.
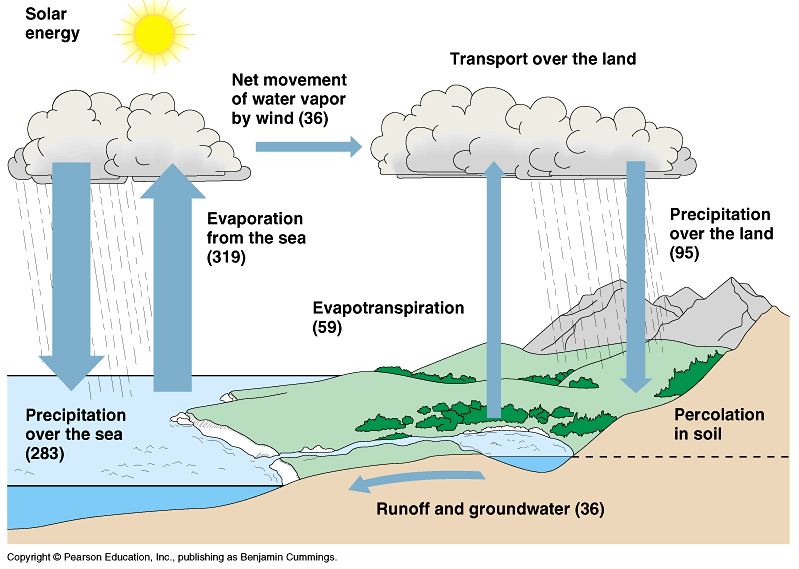
The water cycle is the process by which water travels in a sequence from the air (condensation) to the earth (precipitation) and returns to the atmosphere (evaporation). It is also referred to as the hydrologic cycle.
Human use of water can transform the water cycle through irrigation or the construction of dams, for example.
The Water Cycle The “first” step in the water cycle is when water in oceans, lakes, or other bodies/collections of water is warmed up by the Sun. Heat adds energy to matter. This causes the molecules in the water to move faster and farther apart, until they move so far apart that they become a gas instead of a liquid – the water becomes water vapor. Eventually the water vapour condenses into clouds. This means that the particles in the water vapour slow down and cool off, resulting in them becoming water droplets. These water droplets group together to form clouds. This is why a parachutist unfortunate enough to fall through a cloud would end up covered in water droplets. When enough water droplets (remember, these are usually very small to begin with) form ‘inside’ clouds, they become heavy enough to fall towards the Earth. This is usually observed as rain, but Precipitation can also occur as snow, hail, sleet, etc., depending on temperatures and humidity. Runoff is when water travels across land. Rain will land in the ground, streets, oceans, and in streams and rivers. Eventually it makes its way to a body of water. After a while, the water is evaporated and the water cycle repeats.
Importance Of Carbon Cycle
- Carbon is an essential body component of all living organisms. It is taken into plants in the form of carbon dioxide.
- Carbon dioxide is used by plants along with water, sunlight and chlorophyll for photosynthesis to take place. This is important for production of carbohydrates which plants and animals require for energy.
- Carbon helps to purify and maintain atmospheric level of carbon (IV) oxide.
Importance of water Cycle
- It facilitates the translocation of nutrients to different parts of the plant where they are utilised.
- It provides the medium of absorption of minerals by plant roots.
- It is an essential raw material for photosynthesis.
- It facilitates enzymatic activities occurring in plants.
- It has cooling effect on crops.
- It is a major constituent of plant protoplasm.
- It facilitates opening and closing of stomata.
- It encourages seed germination.
- It maintains plant turgidity.
Importance of Phosphorus Cycle
Phosphorus is an essential nutrient for plants and animals. Phosphorus is a limiting nutrient for aquatic organisms. Phosphorus forms parts of important life-sustaining molecules that are very common in the biosphere. Phosphorus does not enter the atmosphere, remaining mostly on land and in rock and soil minerals.
Importance of Carbon Cycle
It is important for a few reasons:
- Carbon is an essential element for all life, so understanding how it moves helps us to understand biological processes and factors that influence them.
- One form carbon takes is the greenhouse gas carbon dioxide, CO2. Increased levels of carbon dioxide insulate the Earth, causing temperatures to rise. Understanding how carbon dioxide is absorbed and released helps us understand the climate and predict global warming.
- Carbon is not in balance, so it’s important to learn where it is being stored and released. The rate at which carbon is deposited into living organisms is not the same as the rate it is returned to the Earth. There is about 100x more carbon in living matter than in the Earth. Burning fossil fuels releases massive amounts of carbon into the atmosphere and to the Earth.
- The carbon cycle is tied to the availability of other elements and compounds. For example, the carbon cycle is tied to the availability of oxygen in the atmosphere. During photosynthesis, plants take carbon dioxide from the air and used it to make glucose (stored carbon), while releasing oxygen.
Importance of Nitrogen Cycle
Plants and animals could not live without nitrogen. It is an important part of many cells and processes such as amino acids, proteins, and even our DNA. It is also needed to make chlorophyll in plants, which plants use in photosynthesis to make their food and energy.